How to Explain the Chemistry of Cleaning with Vinegar
You have probably heard of making your own multi-purpose cleaning solution using distilled vinegar. You may even use it yourself! But have you ever wondered how (or if) vinegar actually cleans things? Read on to learn the science of cleaning with vinegar, access a simple science activity to do with your kids, and make your own citrus-infused vinegar cleaning solution.

How Does Vinegar Work as a Cleaning Agent?
Distilled vinegar, also known as white vinegar, is a versatile and natural cleaning agent that can effectively clean and disinfect a variety of surfaces. The acidic properties of vinegar make it an effective cleaner for a variety of reasons:
- Dissolving grease and grime: Vinegar can dissolve grease and grime on surfaces due to its acidic properties. It can also remove soap scum and mineral deposits from hard water.
- Neutralizing odors: Vinegar can neutralize unpleasant odors from surfaces, such as kitchen sinks, bathrooms, and laundry rooms.
- Killing germs and bacteria: Vinegar has natural antimicrobial properties, making it an effective disinfectant against some germs and bacteria. Notice I say some, not all. More on that in a moment.
- Non-toxic and eco-friendly: Vinegar is a natural and non-toxic cleaning agent that is safe to use around children and pets. It is also an eco-friendly alternative to harsh chemical cleaners.
To use vinegar as a cleaning agent, it can be diluted with water to create a cleaning solution. A general cleaning solution can be made by mixing one part vinegar to one part water. The solution can be applied to a surface and wiped clean with a cloth or sponge. For tougher stains or soap scum, undiluted vinegar can be used. However, vinegar should not be used on porous surfaces like granite or marble, as the acid can cause damage.
Can Vinegar Be Used as a Disinfectant?
Distilled vinegar can be an effective disinfectant against some germs and bacteria. Vinegar has natural antimicrobial properties that can help to kill or inhibit the growth of some microbes, including E. coli and Salmonella. However, vinegar may not be effective against all types of bacteria, viruses, and fungi.
One of the main components of vinegar is acetic acid, which is what gives it its disinfecting properties. According to some studies, vinegar with 5% acetic acid concentration can kill up to 90% of certain bacteria and viruses. However, vinegar may not be as effective as other disinfectants such as bleach or hydrogen peroxide.
It’s important to note that vinegar should not be used as a disinfectant on surfaces that have come into contact with harmful bacteria or viruses, such as those contaminated with fecal matter or blood. In these cases, a stronger disinfectant, such as bleach or hydrogen peroxide, should be used.
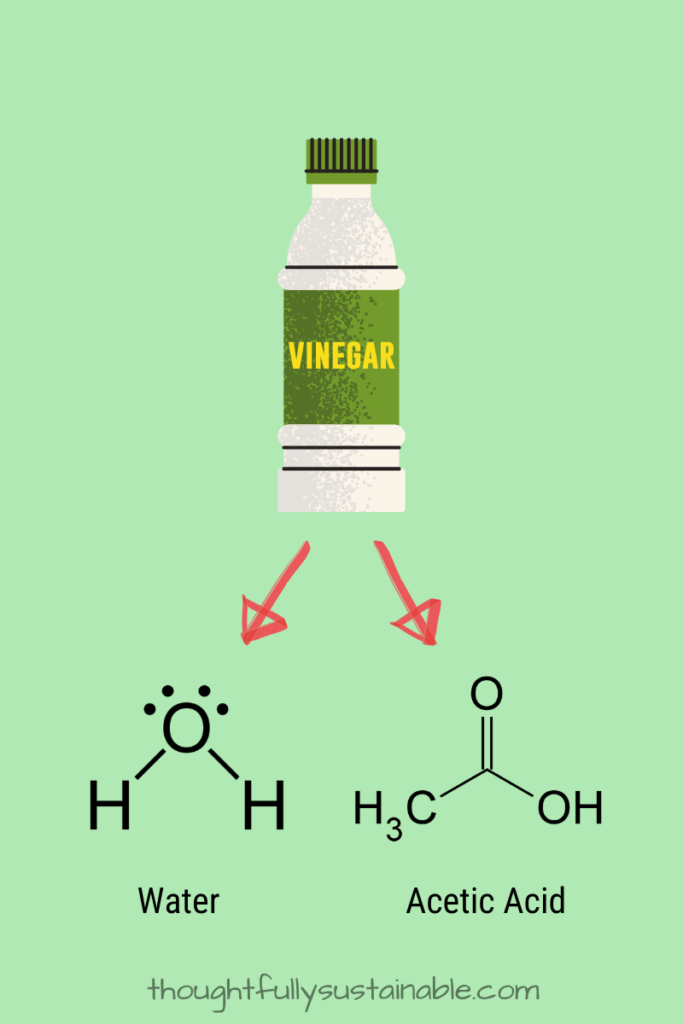

The Cleaning Power of Vinegar: A Chemistry Tale
The most commonly used vinegar to clean is distilled white vinegar. Distilled white vinegar is comprised of 2 main ingredients: water (H2O) and acetic acid (CH3COOH).
Typically, distilled white vinegar contains 5-8% acetic acid, giving it a pH of about 2.5 and thereby classifying the vinegar as an acid. These acid molecules are excellent at lifting dirt and soap scum, along with the calcium carbonate (CaCO3) or lime scale residue that we commonly refer to as hard water build-up. Also, due to its acidity, distilled vinegar is excellent at warding off the growth of mold and bacteria. This is why it’s the solution of choice for pickling your favorite vegetables.
So, what’s pH mean again?
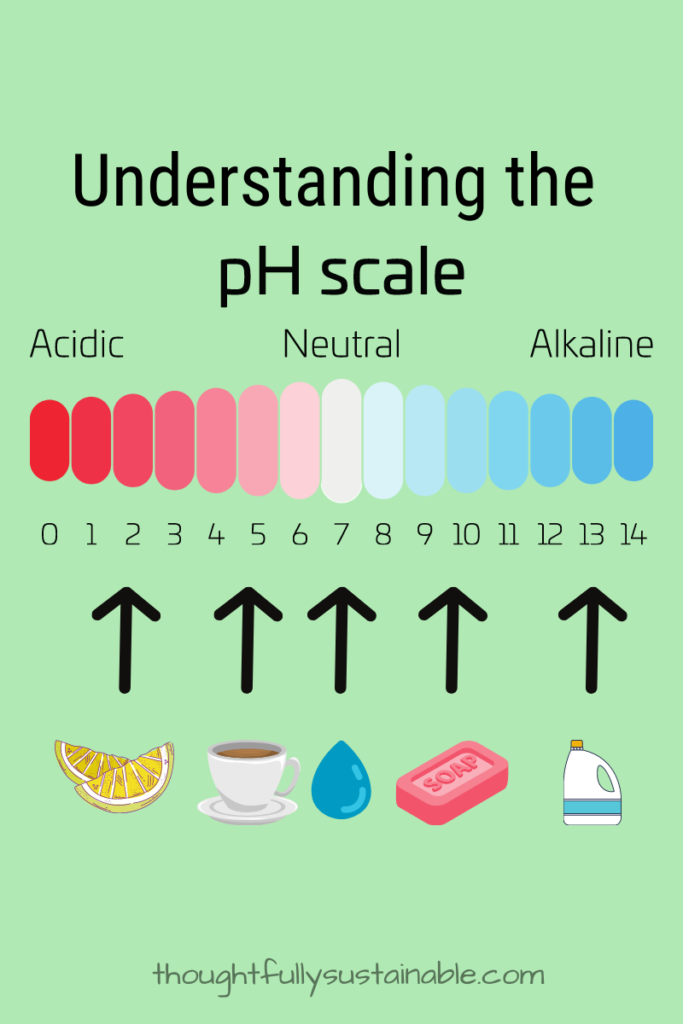

An Overview of Acids, Bases and the pH Scale.
The scale for measuring the strength of acids and bases is referred to as the pH scale, which ranges from 0-14. The closer to 0, the more acidic a substance, the closer to 14 the more basic a substance. Split the difference and have a pH of 7 – that’s neutral.
Since most all-purpose vinegar cleaning recipes call for a 50:50 vinegar to water ratio, this decreases the acidity of the vinegar, raising the pH closer to 5.0. This multi-purpose cleaning solution is still acidic, but not as abrasive as pure distilled vinegar.
To see this for yourself, try out this simple experiment:


The Chemistry of Cleaning with Vinegar Science Experiment
To experiment with the cleaning power of distilled vinegar, you’ll need the following materials.
Materials for the Chemistry of Cleaning with Vinegar Experiment
- 3 dirty coins of the same denomination. I chose 3 pennies, as the copper coating tends to show dirt rather easily.
- 3 shallow dishes
- 1 measuring cup
- Distilled white vinegar
- Filtered water
- Scrap paper and a pen/pencil
- Rag or cloth


Once you’ve collected the supplies, follow these simple instructions.
Instructions for the Chemistry of Cleaning with Vinegar Experiment
- Place 3 dishes in a row and label them A, B and C.
- In dish A, pour ¼ cup (~60 mL) distilled white vinegar.
- In dish B, pour ⅛ cup (~30 mL) distilled white vinegar and ⅛ cup (~30 mL) of water.
- In dish C, pour ¼ cup (~60 mL) water.
- Place a coin in front of each dish and take a photo to record the “before” appearance of each.
- Once photographed, put a coin in each dish and let sit for 24 hours.
- After 24 hours, remove each coin from its dish.
- Using a rag, dry each coin and place in front of its respective dish.
- Take a photo to record the “after” appearance.
- Compare the “before” and “after” photos and record any changes.
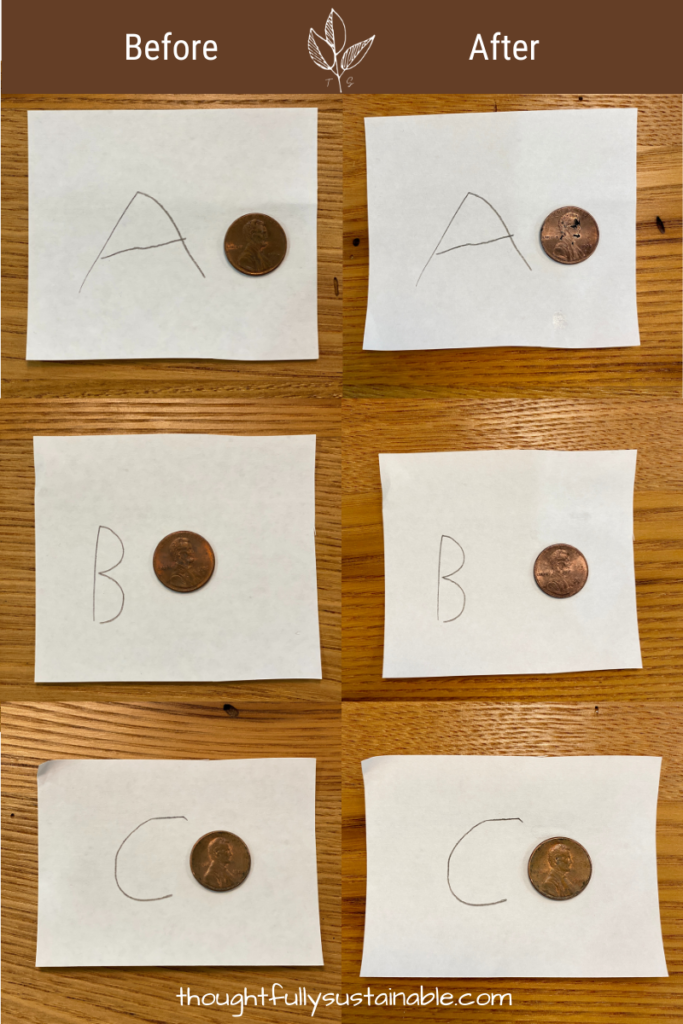
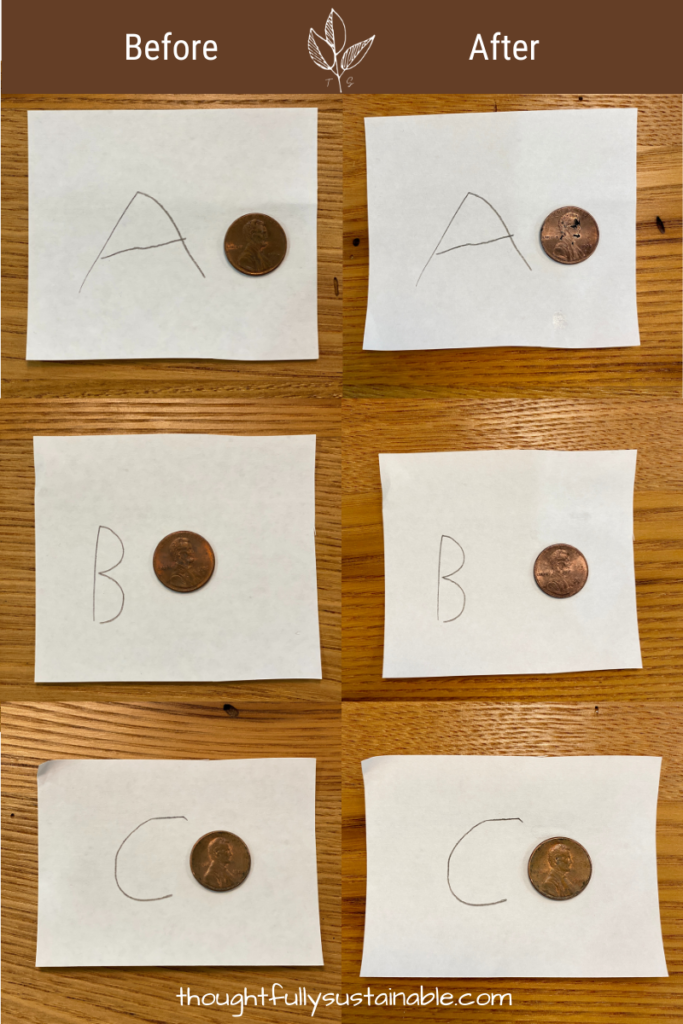
You should discover that the penny in dish A had the most drastic change before and after, while the penny in dish C has little to no change during the experiment.
Free Printable for the Chemistry of Cleaning with Vinegar Experiment
If you’d like a free printable to accompany this experiment, simply click the button below to have a copy delivered to your inbox!

How to Make An Infused Vinegar Cleaning Solution
Now that you’ve discovered how distilled white vinegar can be an effective cleaning solution, are you ready to make your own multi-purpose cleaner without the pungent vinegar smell? Try infusing some distilled white vinegar with your citrus food scraps!
Materials to Make A Citrus-Infused Vinegar Cleaning Solution
To make your own infused vinegar cleaning solution, you’ll need the following materials:
- Distilled white vinegar
- Water
- Citrus peels (orange peels, lemon rind, grapefruit peels, etc.)
- Refillable spray bottle
Instructions to Make A Citrus-Infused Vinegar Cleaning Solution
- Save your citrus peels in a glass jar.
- Once you’ve filled the jar halfway with citrus peels, fill the jar with distilled white vinegar.
- Place the jar in a cool, dark spot (like underneath the kitchen sink) for 2 weeks.
- After 2 weeks, strain the citrus peels from the vinegar and put them in the compost bin.
- In an empty spray bottle, fill half of the bottle with the infused vinegar and the other half with filtered water.
- Mix and get cleaning!
Remember that a vinegar-based cleaning solution can work as a multi-purpose cleaner for surfaces that are not porous and will not be damaged by acidic solutions. A vinegar cleaning solution is not an effective disinfectant solution for all bacteria and viruses.
Additional Green Cleaning Recipes and Techniques
Want to learn more about sustainable cleaning methods?
My friend Nash Gierak has written an excellent e-book titled Clean + Green: Minimalist Sustainable Cleaning. In it, she walks you through reasons to clean green, what basic supplies you’ll need, various cleaning recipes and techniques based on the rooms you’re tackling, as well as a crochet dishcloth pattern and additional resources!
To learn more about Clean + Green: Minimalist Sustainable Cleaning, click here.


Share the Science of Sustainability
Combining the knowledge of chemistry with the power of simple, sustainable cleaning methods is an excellent way to answer the “why” behind sustainable living choices.
If you’ve found this post useful, please Pin It to your favorite Pinterest board, share it on Instagram or Facebook, or forward it to a teacher or parent that’s looking to add some sustainability science to their learners’ lives!







