How to Make a Cartesian Diver Science Project for Kids
Have you ever wondered how you can create an intriguing science experiment using simple materials found around your home? Look no further, as today you’ll learn how to make a Cartesian diver: a fascinating Boyle’s Law experiment. The Cartesian diver offers a hands-on approach to understanding buoyancy, density, and the effect of pressure on gases.
With just a few items, such as a liter bottle, an eyedropper or pipette, and some water, you can create an impressive Cartesian diver that will amaze your students. By changing the pressure inside the bottle, your students will observe the diver rising and sinking, giving them a visual representation of Boyle’s Law in action.
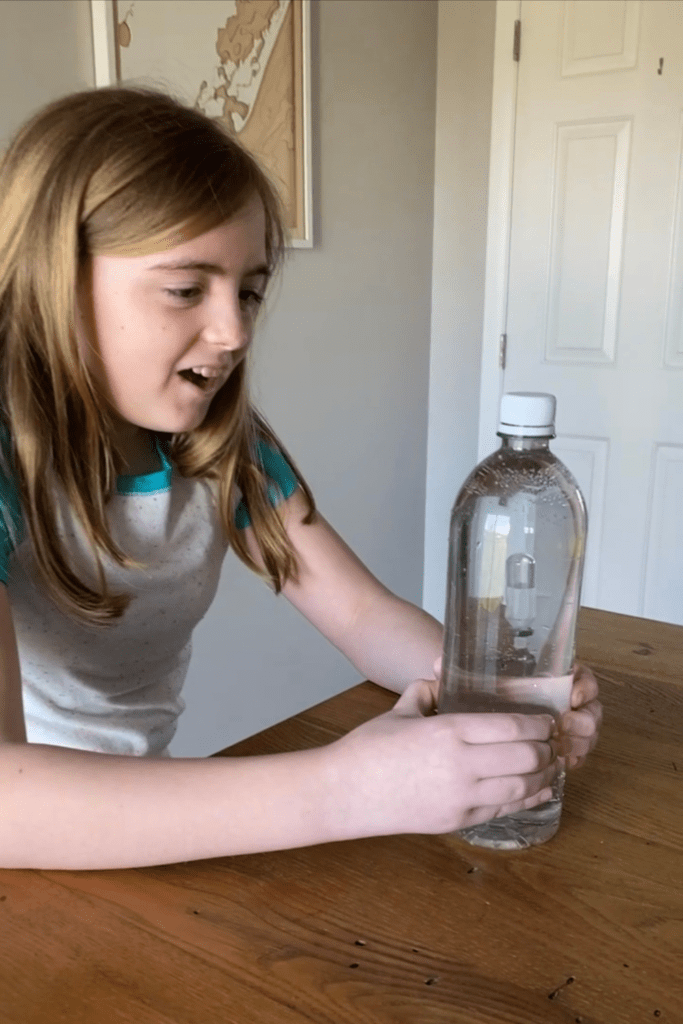
You may have seen a Cartesian diver demonstration before, where a small, air-filled object sinks and floats inside of a water-filled bottle, depending on how hard the sides of the bottle are squeezed. Did you know that this simple experiment can be used to teach multiple chemistry concepts? Perfect for various age groups and abilities, the creation of a Cartesian diver can be used to address Boyle’s Law, buoyancy, Archimedes’ Principle, the reuse of simple household materials, and the practical application of how scuba divers and submarines can alter their buoyancy when submerged in water.




How Does a Cartesian Diver Work?
A Cartesian diver is a simple science experiment that demonstrates the principles of buoyancy and pressure. The experiment involves a small glass or plastic container filled with water and a small object, usually a rubber or plastic eye dropper or pipette, with some air trapped inside it.
When the sides of the bottle are squeezed, the pressure on the water increases, causing the air inside the diver to compress. This decrease in volume of the air causes the overall density of the diver to increase, causing it to sink to the bottom of the bottle.
When the pressure is released, the air inside the diver expands again, increasing its volume and decreasing its overall density. This causes the diver to float back up to the top of the bottle.
The Cartesian diver demonstrates how changes in pressure can affect the buoyancy of an object, and how objects with different densities can float or sink in a fluid.
Where Do Cartesian Divers Get Their Name?
This simple buoyancy demonstration is called a Cartesian diver because it is named in honor of René Descartes, a 17th-century French scientist, mathematician and philosopher who is credited with first creating this cool science visual.


What Does Boyle’s Law Have to Do with a Cartesian Diver?
Boyle’s Law is a fundamental law of physics that describes the relationship between the pressure and volume of a gas, while the temperature remains constant. It is named after the Irish scientist Robert Boyle, who first discovered this relationship in the 17th century.
When we increase the pressure by squeezing the sides of the plastic bottle, we decrease the volume of the air trapped inside the diver. When we decrease the pressure on the plastic bottle by not squeezing the sides, the air trapped inside the diver can expand and take up more space again.
In chemistry terms, Boyle’s Law states that, for a given amount of gas at a constant temperature, the pressure of the gas is inversely proportional to its volume. In other words, if the pressure of a gas increases, its volume will decrease proportionally, and vice versa.
You may come across Boyle’s Law expressed mathematically, which looks like this:
P1V1 = P2V2
where P1 and V1 are the initial pressure and volume of the gas, and P2 and V2 are the final pressure and volume of the gas, respectively.
Boyle’s Law is particularly useful in a variety of practical applications, such as scuba diving, where it is important to understand the relationship between pressure and volume of gases in the body. It also plays a crucial role in the design and operation of many industrial processes, such as chemical reactions, refrigeration systems, and fuel cell technology.


What Does Buoyancy Have to Do with a Cartesian Diver?
You’ll notice that the term buoyancy is also mentioned often when talking about Cartesian divers. But what does the word buoyancy mean?
Buoyancy is the upward force that a fluid, such as water or air, exerts on an object that is partially or completely submerged in it. This force is the result of the pressure difference between the top and bottom of the object due to the weight of the fluid above it.
Buoyancy is governed by Archimedes’ Principle, which states that the buoyant force on an object in a fluid is equal to the weight of the fluid displaced by the object. In other words, an object will float if the buoyant force acting on it is greater than its weight, and it will sink if the weight is greater than the buoyant force.
The buoyant force is proportional to the volume of the object that is submerged in the fluid. This is why objects with larger volumes, such as ships or submarines, are able to float, while smaller objects, such as stones, sink.
Buoyancy plays an important role in many practical applications, such as in the design of boats, ships, and submarines, as well as in understanding the behavior of gases in the atmosphere, and the movement of fluids in pipes and channels. By building your own Cartesian diver, you can see the principles of buoyancy in action in a smaller space.
Video of a Cartesian Diver in Action
Here is a short video to show you how a Cartesian diver works. By varying the pressure around the sides of the bottle with my hands, the diver moves from the top of the bottle to the bottom of the bottle and back again.


Materials to Make a Cartesian Diver Science Project to Demonstrate Boyle’s Law
To make a Cartesian diver, you will need:
- A liter bottle made of clear plastic with a tight-fitting lid
- An eye dropper, medicine dropper, or pipette made of plastic or rubber – if you don’t have any on hand, I like these.
- Small metal nut or a paper clip to fit around the eye dropper of the pipette
- Scissors
- Water
- Optional: food coloring or small objects to add to the water for visual effect
Bottles and containers made of plastic work best because the sides of the bottles can be squeezed to increase the pressure on the water and air inside the container. I mention that 1 liter bottles are preferred because they are usually made of a thicker plastic than a single-use bottle of water. That being said, use what you have and investigate which type of bottle or container works best for you.
If you do not have access to a plastic eye dropper or pipette, you can use a plastic straw, a paper clip and some modeling clay as alternative materials for constructing your Cartesian diver.


Instructions to Make a Cartesian Diver to Demonstrate Boyle’s Law
Here are the steps to make a Cartesian diver:
- Fill the container with water, leaving some room at the top for the diver to move up and down.
- Fill the plastic eye dropper, medicine dropper or pipette with air by squeezing it and then placing it in the water with the open end facing down.*
- Slide a metal nut or a paper clip around the open end of the eye dropper.
- Use scissors to cut the majority of the long, thin portion of the eye dropper off, leaving the air bulb intact.
- Screw the lid tightly onto the container to prevent air from entering or leaving.
- Test the diver by squeezing the sides of the bottle. The diver should sink to the bottom of the bottle when the container is squeezed and rise to the top of the bottle when the pressure is released.
- Optional: you can add food coloring or small objects to the water for visual effect.
Note: If the diver does not sink when you squeeze the container, try adding more water to the container, or squeezing the container more forcefully. If the diver sinks too quickly and does not rise to the surface, try releasing some water from the container to reduce the water pressure.
*If you are using a plastic straw, a paper clip, and modeling clay to construct your diver, follow these alternative instructions: Cut one third of the plastic straw and bend it in half. Open up the paper clip and insert each looped end into each end of the straw. Secure the paper clip to the straw with a small ball of modeling clay.


Practical Applications of Science Learned from the Cartesian Diver Experiment
The Cartesian diver science experiment is a simple yet effective way to learn about buoyancy, pressure, and density, which are fundamental concepts in science and engineering. Here are some practical science applications of these concepts:
- Submarines and ships: The principle of buoyancy is critical in the design and operation of submarines and ships. By controlling the buoyancy of the vessel with ballast tanks, submarines and ships can alter their position in the water.
- Scuba diving and deep-sea exploration: Buoyancy and pressure are also important in scuba diving and deep-sea exploration. By regulating the air in the diving tank and adjusting the buoyancy compensator, divers can control their depth and movement underwater.
- Weather forecasting: Pressure is a key factor in predicting weather patterns. Changes in air pressure can indicate the presence of high or low-pressure systems, which can affect the weather in a particular area.
- Hot air balloons: Hot air balloons rely on the principle of buoyancy to rise in the air. The heated air inside the balloon is less dense than the surrounding air, causing the balloon to rise.
- Aerospace engineering: Understanding buoyancy and pressure is crucial in the design and operation of aircraft and spacecraft. By controlling the air pressure and the density of the surrounding air, engineers can design more efficient and effective aircraft and spacecraft.
Drawing Conclusions from the Cartesian Diver Science Experiment
In this cool science experiment, you have successfully created a Cartesian diver and observed the effects of Boyle’s Law in action. By using simple materials such as a plastic bottle, water, and an eye dropper, you have demonstrated how changes in pressure affect the buoyancy of an object submerged in water.
Now that you understand the principles behind a Cartesian diver, you can use this knowledge to further explore the behavior of gases and liquids under different conditions. This experiment can also serve as a foundation for discussing more complex concepts related to pressure, buoyancy, and other fluid mechanics principles in your classroom.
Experiment with variations of the Cartesian diver or try different materials to observe any changes in the diver’s behavior, such as replacing fresh water with salt water. It can be an excellent way to engage students and generate discussions on the scientific concepts at play. Emphasize the importance of curiosity and exploration, essential for fostering a passion for science and learning.
Continue to challenge yourself and your students with more experiments and projects, deepening your understanding of the fascinating world of physics and chemistry. The Cartesian diver serves as an excellent starting point for your further explorations of fluid mechanics and the natural world around us.
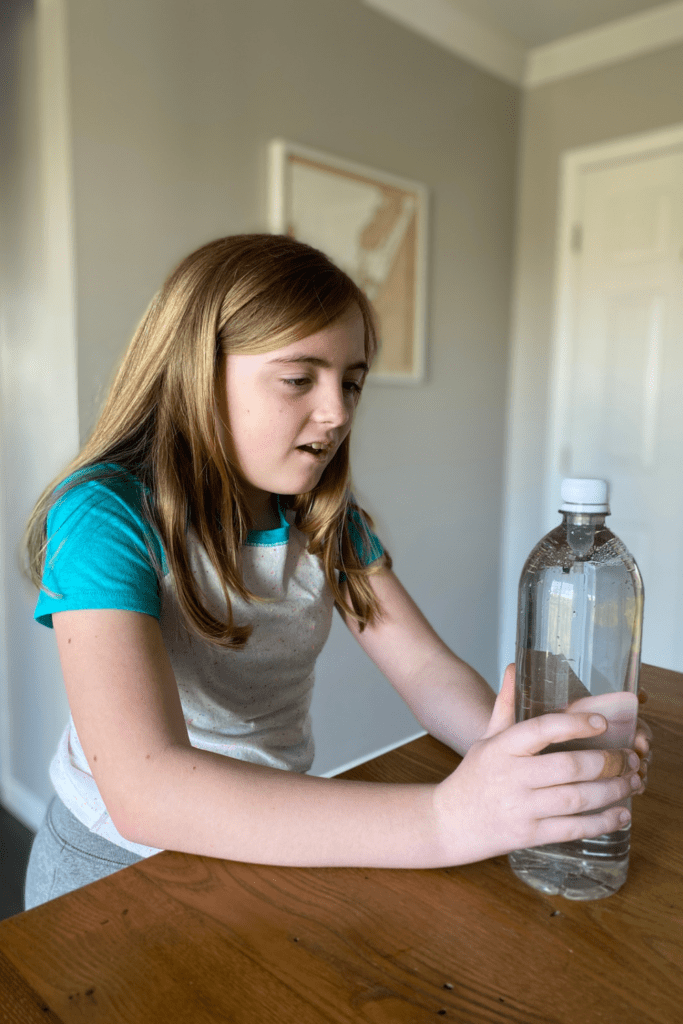
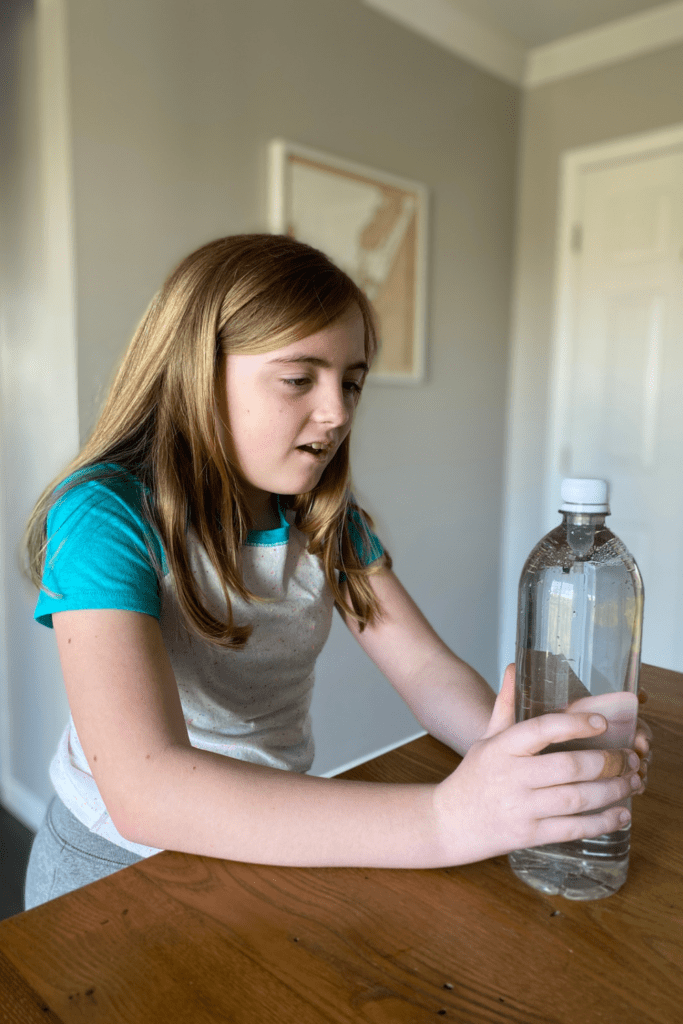
How to Responsibly Dispose of Your Cartesian Diver
Once you are done playing with your cartesian diver, here is a list of ways to responsibly dispose of your project:
- Use the water contained inside the plastic bottle to water indoor houseplants or an outdoor garden.
- Repurpose the plastic water bottle for another amazing science project, such as the landfill learning experiment.
- Recycle the water bottle. Double check to make sure that your recycling center takes the particular type of plastic that the bottle is made of.
- Empty the eye dropper and reuse it for other science experiments or for adding food coloring or paint to art projects.
- Pass the cartesian diver onto a friend and teach them how to use it and the science behind it!
Responsibly repurposing or disposing of your science experiment materials is always the right thing to do and can save you time and money on future investigations.
How to Make a Cartesian Diver: A Boyle’s Law Experiment


In need of a hands-on science experiment that explores buoyancy, Boyle’s Law, and Archimedes’ Principle? Make a simple Cartesian diver out of a few household materials!
Materials
- A clear plastic bottle or container with a tight-fitting lid - I find that 1 and 2 liter soda bottles work well, as do clear plastic shampoo bottles
- An eye dropper or pipette made of plastic or rubber
- Small metal nut to fit around the eye dropper of pipette
- Water
- Optional: food coloring or small objects to add to the water for visual effect
Instructions
- Fill the container with water, leaving some room at the top for the diver to move up and down.
- Fill the eye dropper or pipette with air by squeezing it and then placing it in the water with the open end facing down.
- Attach a metal nut or paper clip around the thin portion of the plastic eye dropper and cut the remaining portion of the eye dropper off with scissors.
- Screw the lid tightly onto the container to prevent air from entering or leaving.
- Test the diver by squeezing the sides of the container. The diver should sink when the container is squeezed and rise when the pressure is released.
- Optional: you can add food coloring or small objects to the water for visual effect.
Notes
Bottles and containers made of plastic work best because they can be squeezed to increase the pressure on the water and air inside the container. I mention that 1 and 2 liter bottles are preferred because they are usually made of a thicker plastic than single use plastic water bottles. That being said, use what you have and investigate which type of bottle or container works best for you.
Note: If the diver does not sink when you squeeze the container, try adding more water to the container, or squeezing the container more forcefully. If the diver sinks too quickly and does not rise to the surface, try releasing some water from the container to reduce the water pressure.








2 Comments
Comments are closed.