How is Plastic Made? Understanding the Science of Plastic Production
We are surrounded by plastics in every aspect of our lives, from the consumables we purchase to the clothes we wear to the cars we drive. Read on to learn the science of how these ubiquitous materials are made and the role petroleum plays in the production of plastic.

Like it or not, plastics are a part of our daily lives. I feel we’ve all grown to realize that vilifying one another for utilizing this disposable petroleum-based product is counter-productive. Instead, our energies should be collectively focused on supporting the communities that are most negatively affected by the pollution associated with the production of plastics, as well as the negligent way in which plastics are disposed of.
By assessing the plastic that enters our homes and curbing our consumption of that which is avoidable is a great place to start. In this post, I’ll be discussing the chemistry behind the production of various plastics in an effort to bring the science of petroleum processing into mainstream conversations.
Please note that my intention is not to demonize plastics, as this lightweight, durable material is essential in many areas and, in some, downright life-saving. My purpose is to explain the role petroleum plays in the manufacture of this product.
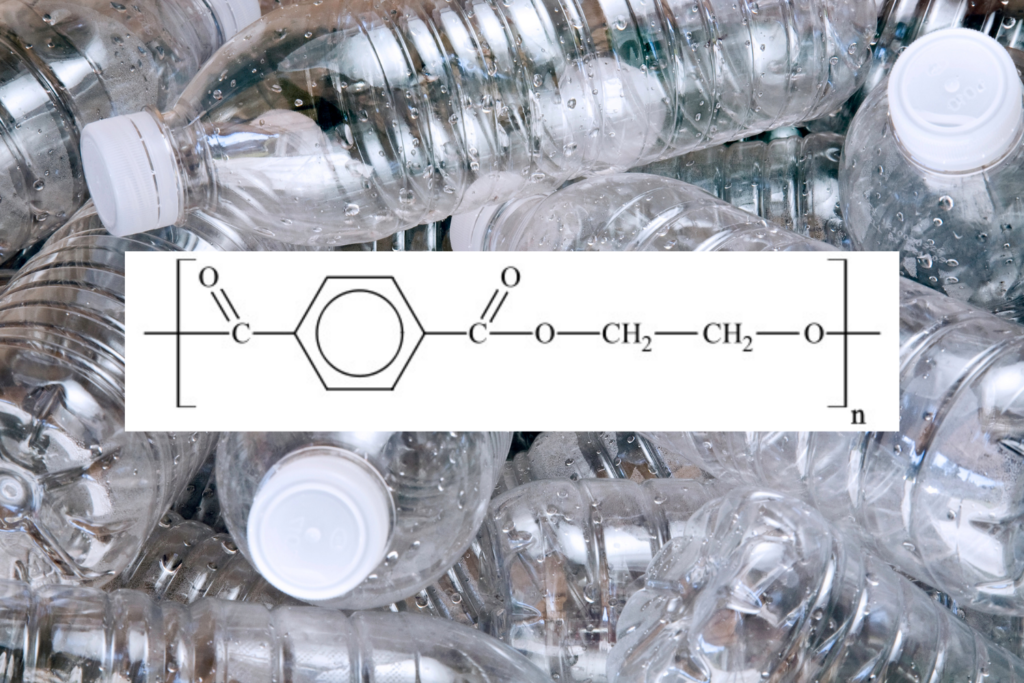
The Building Blocks of Plastics: Monomers and Polymers
It all starts with a single unit, called a monomer.
In order to make plastics, thousands of identical carbon based monomers are linked together to make a polymer, which simply translates into “many units”.

So, if we were to use the above image as a model, 1 paper clip represents a monomer, while the string of paper clips is a polymer.
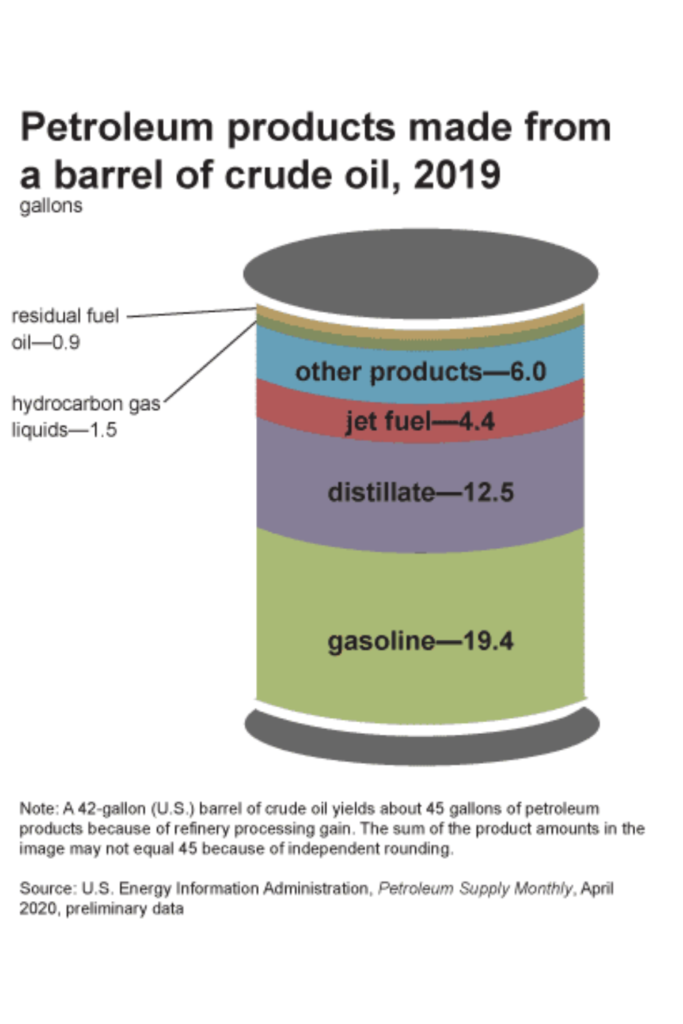
The carbon needed to create these polymers comes from refining petroleum. In the U.S., for every 42 gallon barrel of crude oil that’s refined, 6 gallons are used to create “other products”, in which plastics are one.
The prefix poly- shows up in the naming of all types of plastics, and not just the packaging kinds. Think polyester, polyvinyl chloride (PVC), polyethylene (plastic bags), polystyrene (Styrofoam) and polytetrafluoroethylene (Teflon).
These polymers can be used to make 2 types of plastics: thermoplastics or thermosets:
- Thermoplastic can be heated to soften and reform, then cooled to harden again, making this type of plastic recyclable.
- Thermoset cannot be melted and reformed. Examples include car parts, insulators and circuit breakers.

From Oil to Nurdles
Synthetic plastics are made from fossil fuels.
In order to transform those underground carbon molecules into plastics, a few things need to happen:
- Crude oil or natural gas is extracted from the Earth.
- The oil or gas is transported via pipeline or tanker to a petroleum refinery.
- At the refinery, the crude oil is heated in a furnace and then distilled in order to separate the different oil components. You’ll commonly hear this process referred to as fractional distillation, because it separates the crude oil into different fractions, based on the weights and boiling points of each component.
- The third “fraction” to be distilled is called naphtha, which are molecules that are between 5-10 carbons long. This is what is used to create plastics.
- The naphtha is further processed via cracking, which breaks the longer carbon chains into smaller carbon chains (a.k.a monomers). Cracking is done by using fractions from natural gas distillation.
- The monomers are then linked together in a process called polymerization to make polymers. These polymers resemble thick strings of taffy and are called resins.
- These strings of resins are then ground up into pellets, called nurdles, which are shipped off to be made into all sorts of plastic products.
Even while writing this, I am still dumbfounded by one thing – how much energy and infrastructure is necessary to make the raw materials for plastic. And we haven’t even touched on the production, distribution, consumption and disposal of these plastic products, yet.


Plastic: A Lucrative Byproduct
Jet fuel, gasoline, propane, diesel fuel and kerosene all come from the same source – crude oil. So do the raw materials for plastic.
I mentioned earlier that in order to extract these different fuels from crude oil, the oil is separated by heat through a process called fractional distillation.
One of the byproducts of this process is called naphtha, which is a group of carbon atoms anywhere from 5-9 carbons in length that boils at 70 ℃ (158 ℉). This seemingly useless group of carbon atoms became a hot commodity when it was discovered that naphtha could be further broken apart and made into the product we now call plastic.
Essentially, plastic is the icing on the cake for oil companies.
Big oil has taken notice that our collective awareness has grown for the negative implications that burning fossil fuels has for our health and the health of our environment. So what do they do? Build more petrochemical plants to make plastics.
Quoting the documentary, The Story of Plastic, they’ve simply “found a new way to keep fossil fuels flowing through our economy” . Due to the recent boom of new petrochemical plants, the World Economic Forum predicts that plastic production will double in the next 20 years.

Plastic Production and Human Health
The extraction of natural gas via fracking and the refining of crude oil release numerous chemicals into the surrounding environment.
Here are a few, along with some of their documented effects on human health:
- Benzene – affects the bone marrow and can cause a decrease in red blood cells, leading to anemia. (1)
- Toluene – causes headaches, dermatitis, insomnia and kidney damage. (2)
- Ethyl benzene – causes respiratory problems and, at a chronic exposure level, damages the liver and kidneys. (3)
- Styrene – affects the nervous system (1)
- 1-3 butadiene – affects the nervous system (2)
- Hydrogen sulfide – causes tremors, convulsions, headaches and seizures (1)
- Cyanic acid – causes headaches, vomiting, seizures and an irregular heart beat (1)
References: (1) CDC (2) OSHA (3) EPA
And who are most likely to live near extraction sites and refineries, thus being most affected by these human health hazards?
BIPOC communities.
If you’re interested in learning more about how the health of fenceline communities are being adversely affected by the pollution created from the production of plastic, I highly recommend watching the documentary, “Mossville – When Great Trees Fall.”

Is Silicone a Plastic?
Silicone is made of base chains of silicon and oxygen, which is then bonded under high heat with fossil fuel derived hydrocarbons.
Basically, silicone is a hybrid of synthetic rubber and plastic polymers. So, no it’s not officially a plastic, but it’s made with a lot of the same petroleum based stuff that plastic is composed of!
Silicone shouldn’t be confused with silicon dioxide or silica, which is a naturally derived compound that is the primary component of sand.
Silicone can be found in everything from apparel, food storage and prep products, electronics, voltage line insulators, car parts and household sealants.

What’s so Bad about PVC?
Recently, I saw a tag attached to a lunchbox while browsing the countless aisles of back to school supplies. The tag read ”PVC-free”. I thought it would be a good idea to talk about what PVC actually is.
PVC stands for polyvinyl chloride. The poly- means that it’s a polymer (or long chain) made of vinyl chloride molecules.
Vinyl chloride is a known animal and human carcinogen that specifically targets the liver. And it’s that chloride part that’s the most dangerous.
When chlorine-based molecules are made, used or burned, they create dioxins. The EPA has stated that there is no safe level of dioxins in the human body; it’s at the top of the list of “most toxic chemicals ever produced”.
Exposure to vinyl chloride can occur via water and air that surrounds PVC manufacturing and processing plants, as well as landfills and hazardous waste sites. Chemical safety officials also worry about human exposure to vinyl chloride via water that travels through PVC pipes, as well as food and beverages packaged in PVC laden materials. [Reference]
I’m not a chemical engineer or health expert, so I can’t tell you the likelihood that you’ll suffer from health issues due to exposure to vinyl chloride or the dioxins it produces when burned. But I am a former chemistry teacher, so the least I can do is inform you about what this tag is telling you.
With that knowledge, maybe we can collectively make better decisions about our consumer habits, as well as our support for elected officials that turn a blind eye to the dangers of plastic production and manufacturing plants.